Currently out of stock
Contact us >
This product is only licensed for sale in the Republic Of Ireland
Active Ingredient: Inactivated Leptospira borgpetersenii serovar Hardjo
Target Species: Cattle
Treats and Controls: Leptospiia hardjo, Leptospira interrogans serovar hardjo (hardjo Prajitno) and L. borgpetersenii serovar hardjo (hardjo Bovis)
Administration Method: Subcutaneous injection (under the skin)
Withdrawal Time: There is no withdrawal time for this product
Dosage for cattle: 2 ml per animal. After the initial vaccination a second dose should be given no less than 4 weeks and no more than 6 weeks after.
| Body Weight | Dose Volume | Doses Per Pack |
| All Sizes | 2ml | 25 |
Always read the label and all enclosed information for Spirovac before administering to animals!
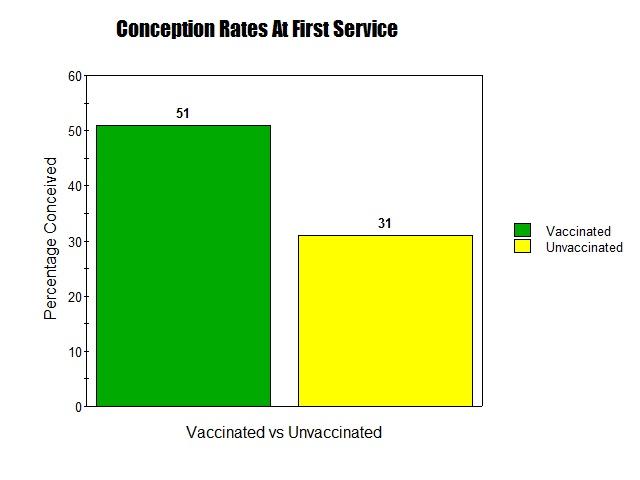
Click here to Download Data Sheet
Summary of Product Characteristics
1 NAME OF THE VETERINARY MEDICINAL PRODUCT
Spirovac
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each dose (2 ml) of Spirovac contains the following:
Active substances:
Inactivated Leptospira borgpeterseniiserovar Hardjo ≥2RP* * RP = ELISA Relative Potency.
Adjuvant
Aluminium hydroxide3.0 to 3.6 mg of aluminium
Excipients
Formaldehyde<1 mg Thiomersalmax 0.01% (w/v) See Section 6.1 for full list of excipients.
3 PHARMACEUTICAL FORM
Suspension for injection.
Slightly coloured turbid liquid which might contain a loose sediment.
4 CLINICAL PARTICULARS
4.1 Target Species
Cattle from 4 weeks of age.
4.2 Indications for use, specifying the target species
For active immunisation
- of cattle to reduce kidney colonisation and shedding of Leptospira borgpetersenii serovar Hardjo type hardjobovis to the extent that no viable organisms can be detected by culture in the urine of vaccinated animals after challenge; a 3 weeks onset of immunity and 12 months duration of protection have been demonstrated by challenge with Leptospira borgpetersenii serovar Hardjo type hardjobovis.
- of cattle persistently infected with Leptospira borgpetersenii serovar Hardjo type hardjobovis: to reduce urinary shedding of Leptospira borgpetersenii serovar Hardjo type hardjobovis withoutclearance of renal colonisation; this effect appears 4 weeks post vaccination and its duration is unknown.
The epidemiological significance of the reduced shedding has not been demonstrated.
The vaccination may not prevent abortion in cows in which placental infection has already occurred.
4.3 Contraindications
None.
4.4 Special warnings for each target species
Vaccinated cattle may be positive in diagnostic tests for leptospirosis and therefore unacceptable for export to some countries.
4.5 Special precautions for use
Special precautions for use in animals
None.
Special precautions to be taken by the person administering the veterinary medicinal product to animals Even though animals may have been vaccinated, the risk of transmission of leptospirosis from cattle to their handlers, albeit very much reduced, remains.
Appropriate precautions should be maintained at all times and prompt medical advice sought in the event of clinical signs of possible infection.
4.6 Adverse reactions (frequency and seriousness)
A diffuse and oedematous swelling up to 10 cm in diameter, sometimes sensitive to palpation, could occur in up to 40% of animals and can last for up to 66 days after completion of vaccination.
The reaction to subsequent vaccinations and the reaction in pregnant animals (see 4.7) are more marked.
The injection site reaction may be sensitive to palpation the week following vaccination and may persist as a hard nodule for several weeks.
4.7 Use during pregnancy, lactation or lay
Can be used during pregnancy and lactation.
The swelling is more marked in pregnant animals.
A diffuse swelling of up to 22 cm in diameter can occur following second injection.
This effect is more marked for pregnant animals in their third trimester of pregnancy.
4.8 Interaction with other medicinal products and other forms of interaction
No information is available on the safety and efficacy of this vaccine when used with any other veterinary medicinal product. A decision to use this vaccine before or after any other veterinary medicinal product therefore needs to be made on a case by case basis.
4.9 Amounts to be administered and administration route
Dose: 2 ml Administration:
By subcutaneous injection, preferably in the neck.
Shake the container well before withdrawing the dose.
Basic vaccination scheme: 2 doses of vaccine separated by a 4 to 6 week interval.
Revaccination scheme: A single 2 ml dose on an annual basis.
4.10 Overdose (symptoms, emergency procedures, antidotes), if necessary
No adverse drug reactions, other than those listed in Section 4.6, occurred after administration of twice the normal dose.
As part of the natural response following vaccination, and following an overdose of twice the maximum dose of the vaccine, a reactive lymphadenopathy in the local lymph node as well as production of a subcutaneous, granulomatous, inflammatory reaction could be visible under the skin for at least 2 months.
The total duration of this reaction in the underlying tissues is not known.
4.11 Withdrawal period(s)
Zero days.
5 PHARMACOLOGICAL or IMMUNOLOGICAL PROPERTIES
To stimulate an active immunity against Leptospira borgpeterseniiserovar Hardjo type hardjobovis.
Vaccination induces humoral antibody response and cell-mediated immunity as measured by serology and gamma-interferon production.
A marked, statistically significant difference is also seen in the anamnestic response following a single booster vaccination or infection (challenge) 12 months after primary vaccination.
A strong serological cross-reactivity post vaccination has been demonstrated against Leptospira interrogansserovar Hardjo, a closely related species in the same serovar.
This was sustained for at least 12 months following primary vaccination, and is also seen in the anamnestic response following a single booster vaccination.
A cattle challenge model is not available to document protection.
ATC Vet code:
QI02AB03 (Immunologicals for bovidae, inactivated bacterial vaccines, Leptospira).
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Aluminium hydroxide Thiomersal Formaldehyde Water for injections
6.2 Incompatibilities
Do not mix with any veterinary medicinal product.
6.3 Shelf-life
Shelf life of the veterinary medicinal product as packaged for sale: 2 years.
Shelf life after first opening the immediate packaging: 10 hours.
6.4 Special precautions for storage
Store and transport refrigerated (2°C- 8°C).
Do not freeze.
Protect from light.
6.5 Nature and composition of immediate packaging
Container:
5 doses (10ml) or 25 doses (50ml)
Glass type I vials.
Closure:
Chlorobutyl stopper with aluminium overseal.
Outer Packaging:
Cardboard carton with package insert leaflet.
Pack sizes:
5 or 25 doses (10 or 50ml).
Not all pack sizes may be marketed.
6.6 Special precautions for the disposal of unused veterinary medicinal product or waste materials derived from the use of such products
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal products should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
Zoetis Belgium S.A.
2nd Floor,
Building 10
Cherrywood Business Park
Loughlinstown
Co Dublin
Ireland
8 MARKETING AUTHORISATION NUMBER(S)
VPA10387/065/001
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 14th March 2008
Date of last renewal: 28th October 2011
10 DATE OF REVISION OF THE TEXT
September 201
Cattle Injectables
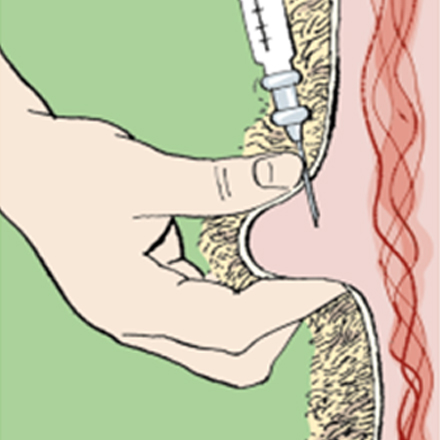
Injectables should be given according to the manufacturer’s instructions at the recommended injection site.
• Always use a clean, sterile syringe and needle. If using a multiple injection gun, ensure the needle is disinfected between injections, e.g. with an automatic sterilisation system.
• If the site to be injected is dirty, clean the skin and swab with an alcohol-impregnated wipe or cotton wool.
• Before injecting, check the expiry date and read the instructions of the product to be used. Some products need to be shaken before use.
• Use the correct-sized needle according to the size of the animal and site of injection.
• Ensure the animal is adequately restrained before attempting the injection.
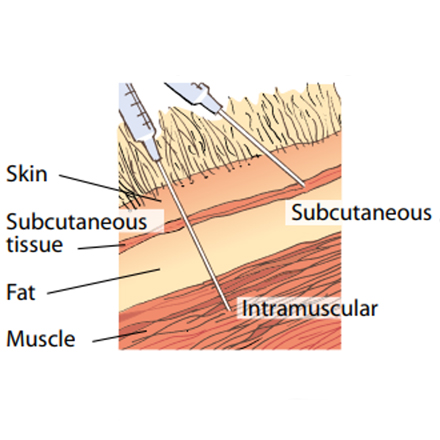
• Take care to ensure it is given subcutaneously and not intramuscularly. Raise a fold of skin at the injection site (mainly neck but some are ear) recommended by the product manufacturer and inject carefully into the space created.
• If a large dose is to be delivered, it may be advisable to split the dose between two injection sites. After the injection, briefly massage the site to improve the dispersal of the injected material.
• Dispose of the needle and syringe in appropriate clinical waste and sharps containers.
Would you like to send this voucher to the recipient via email?
Yes No